Freyr 中国向您致以诚挚问候
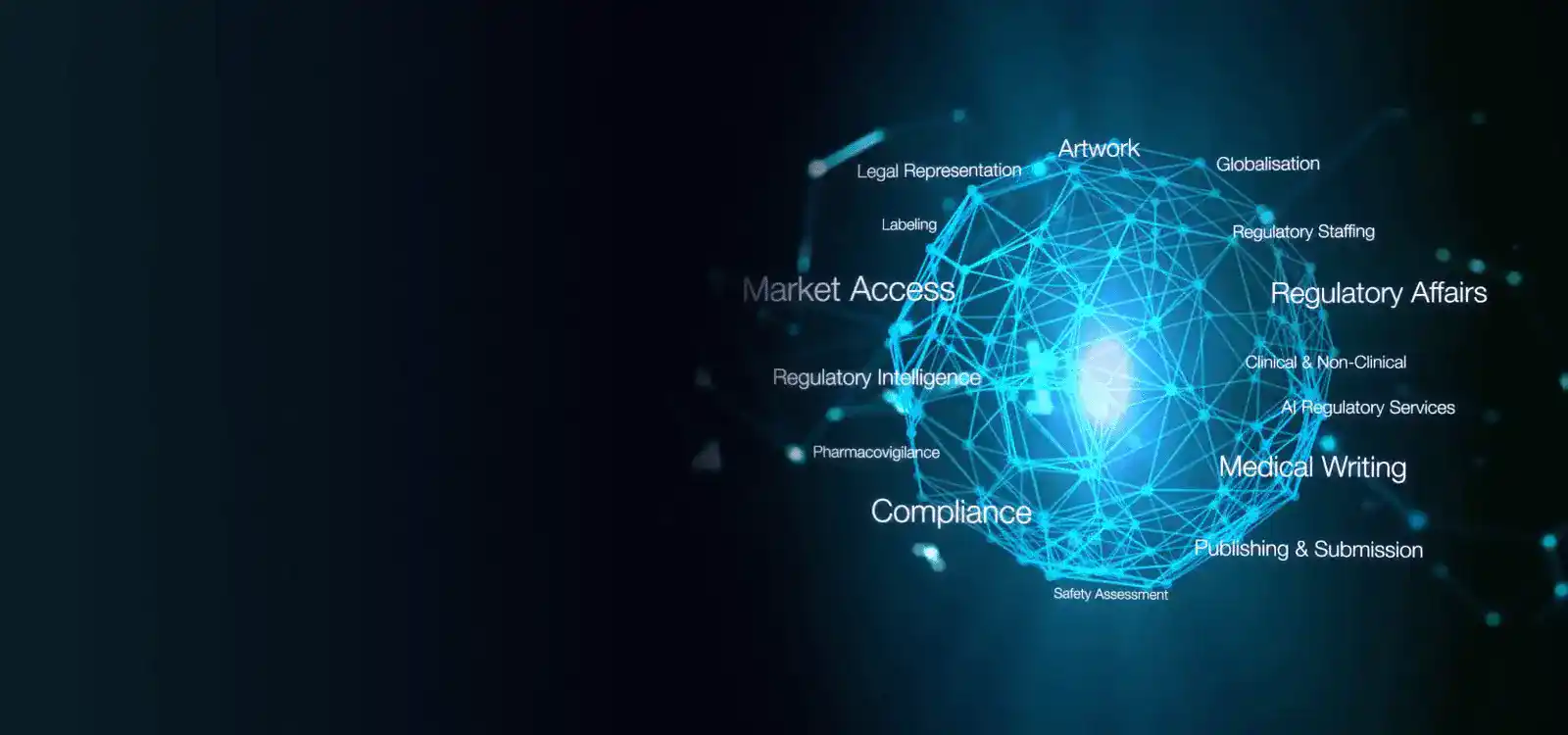
Freyr 中国向您致以诚挚问候 Freyr 是一家领先的法规解决方案提供商,为生命科学企业提供涵盖全法规领域的综合服务。我们在全球 120 多个国家拥有强大的业务网络,助力生命科学企业实现“从本土到全球”的跨越。在中国,Freyr 中国在协助企业完成国家药品监督管理局(NMPA)产品注册方面发挥着关键作用。我们的服务包括法规战略制定、市场情报、国内代表服务、上市后监督(PMS)功能等。此外,我们还提供尖端的法规软件解决方案,旨在简化整个 NMPA 注册生命周期。
探索我们为制药、生命科学、消费品等行业量身定制的解决方案。我们的专业知识涵盖多个行业,确保合规与卓越。探索创新策略与服务,助力您在竞争激烈的市场中脱颖而出。
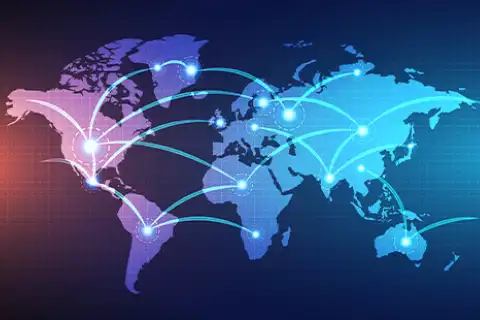
全球覆盖
25+ 国家2200+
名内部法规专家团队持续扩大支持
120+ 国家业务1950+
家全球客户持续增长